Faculty of Chemistry, Iran University of Science and Technology(IUST)
○Azadeh Tadjarodi Nooshin Hafezinejad
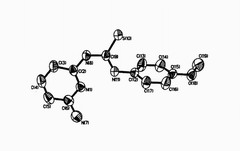
Thioheterocyclic compounds are very interesting ligands because they are able to coordinate to many metals and biological materials by their sulfur and nitrogen atoms. These compounds show potent anti-HIV activity and biological activity and also are suitable for fabrication of selective membrane sensors for monitoring low level of lanthanids. In this work, the reaction between 2,6-diamino pyridine and 4-methoxy phenyl isothiocyanate in 95% EtOH led to formation of N-2-(4-picolyl)-N'-(6-amino phenyl) thiourea. On cooling and reducing the volume, white powder formed. The result compound was characterized by IR, 1H, 13C-NMR spectra, elemental analysis and single crystal X-ray diffraction. We obtained crystals of compound by recrystalization of this powder in ethanol and acetone solution (1:1). The crystal system of this compound is monoclinic with space group P21/c and 4 molecules per unit cell. The intramolecular and intermolecular hydrogen bonding interactions of this compound was confirmed by 1H-NMR and single crystal X-ray diffraction.
