Structural Biology Research Center, PF, IMSS, KEK, Present address: Graduate School of Arts and Sciences, The University of Tokyo* Graduate School of Pharmaceutical Sciences, Kyoto University, Japan** Structural Biology Research Center, PF, IMSS, KEK, Japan***
○Tomoo Shiba* Hiroshi Koga** Hye-Won Shin** Masato Kawasaki*** Ryuichi Kato*** Kazuhisa Nakayama** Soichi Wakatsuki***
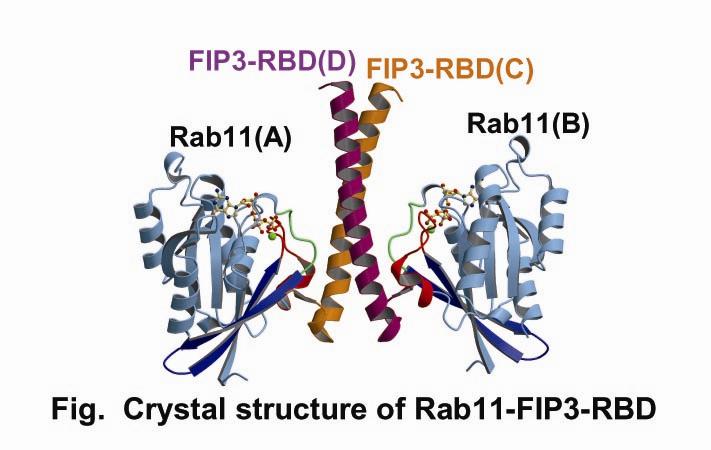
Small GTPases belonging to the Ras-like superfamily regulate intracellular membrane trafficking, and ARF and Rab family members participate in multiple stages of trafficking along the exocytic and endocytic pathways. Rab11 is a well-characterized regulator of endocytic and recycling pathways. Recently, a novel family of Rab11-interacting proteins (FIPs) has been identified. FIPs share a highly conserved ~20-amino acid region, termed Rab11-binding domain (RBD), at their C-termini. FIP3/Arfophlin-1 is a dual effector for Rab11 and ARF5/ARF6 involved in membrane delivery from recycling endosomes to the plasma membrane during cytokinesis. Here, we determined the crystal structure of Rab11 in complex with the FIP3-RBD. The structure reveals that the long amphiphilic α-helix of the FIP3-RBD forms a parallel coiled-coil homodimer, with two symmetric interfaces with two Rab11 molecules (Fig). The hydrophobic side of the RBD helix is involved in homophilic dimerization and mediates the interaction with the Rab11 switch 1 region, whereas the opposite hydrophilic side interacts with the Rab11 switch 2 and is the major factor contributing to the binding specificity.
