00252
Isostructural relationship of (Rb2H2O)C2O4 to (Tl2)C2O4: substitution of one water molecule for two lone electron pairs
Earth Evolution Sciences, Life and Environmental Sciences, University of Tsukuba
○Takuya Echigo Mitsuyoshi Kimata
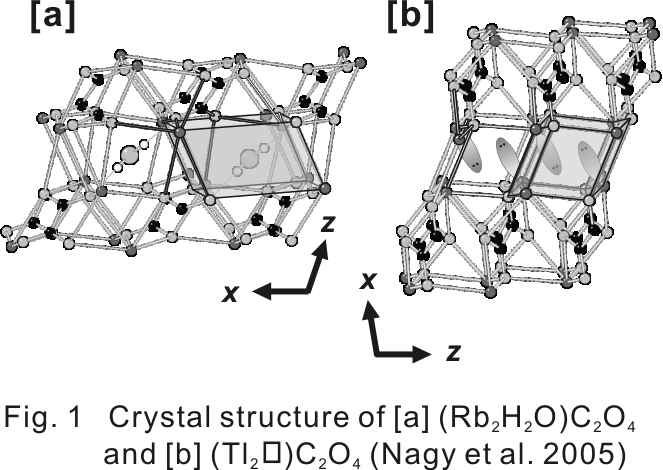
The crystal-chemical and mineralogical significance of the substitution between water molecules (H2O)0 and lone electron pair (LEP) were discussed. Fig. 1 shows that (Rb2H2O)C2O4 is basically isostructural with (Tl2)C2O4. Comparison between interlayer spaces occupied by (H2O)0 and LEP in these two structures revealed the first uncharged substitution: (H2O)0 <=> 2 x LEP, where the directions of the latter occupants are opposite to each other, and nearly perpendicular to a dipole direction of the former. Two LEPs of Tl+ work like a dipole moment of the (H2O)0 in (Rb2H2O)C2O4.
Large and hard cations (K+, Rb+, Cs+ and Sr2+) commonly constitute part of hydrous aluminosilicate minerals, the structures of which suggest that incorporation of (H2O)0 into their crystal structures stabilizes their minerals. On the other hand, soft cations with LEP (Tl+, Pb2+, Sn2+, Sb3+, and Bi3+) are incorporated mainly into sulfide minerals and not into silicate ones, and the former crystal structures suggest that sulfur anion can moderate the polyhedral distortion caused by the LEP. In conclusion, (H2O)0 and LEP play key roles in forming the crystal structures of minerals containing nonvolatile incompatible elements, occurring at the very end of magmatism.