[1] H. L. Carrell et al. (1984) J. Biol. Chem. 256, 3230-3236. [2] G. K. Farber et al. (1987) Protein Eng. 1, 459-466.
Graduate School of Fundamental Life Science, Kitasato University* School of Science, Kitasato University**
○Masanori Ootaki* Hiroyuki Konosu** Hideaki Tagusagawa* Shigefumi Yamamura** Yoko Sugawara**
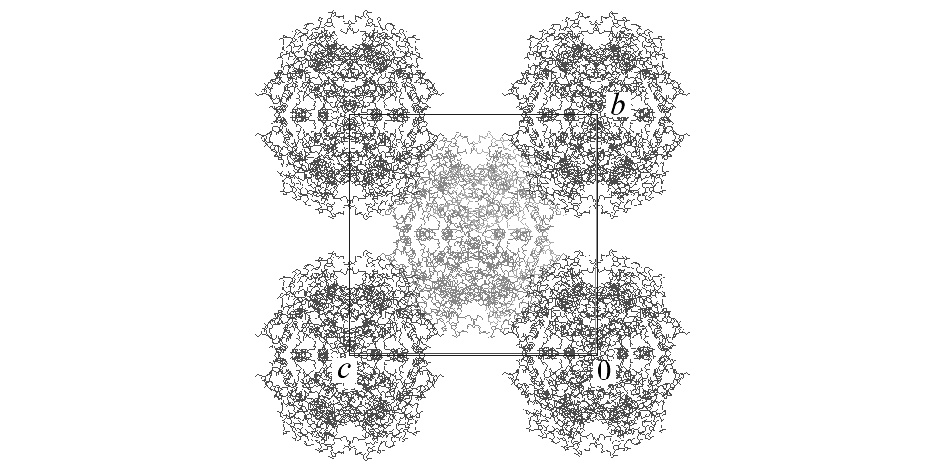
There have been several reports on water-mediated crystal transformations of proteins, which offer valuable information on the role of hydration in crystals. D-xylose isomerase from Streptomyces olivochromogenes form a tetramer and crystallizes in the orthorhombic system with space group of I222 (crystal A) or P21212 (crystal B) [1,2]. We found that the single crystal transformation of crystal A proceeds at 84% relative humidity. The estimated water content was reduced from 56% to 46% associated with the transformation. Precession photographs showed that the space group changed from I222 to P21212. The changes of cell parameters a, b, and c are approximately 1%, 15%, and 3%, respectively. The cell parameters after the transition are similar to those of crystal B. Large shrinkage along the b axis is understandable from the crystal packing of crystal A (Figure 1 based on the PDB data of 1XIB). The transformation from I222 to P21212 would be explained by rotational displacement of tetramers at (0,0,0) and (1/2, 1/2, 1/2) to the opposite direction around the two fold axes parallel to the c axis.
[1] H. L. Carrell et al. (1984) J. Biol. Chem. 256, 3230-3236. [2] G. K. Farber et al. (1987) Protein Eng. 1, 459-466.
