References:
(1) Gonnade, R. G.; Shashidhar, M. S.; Bhadbhade, M. M. Chem. Commun. 2004, 2530.
(2) Manoj, K.; Gonnade, R. G.; Bhadbhade, M. M.; Shashidhar, M. S. Cryst. Growth & Des. 2006, 6, 1485.
Center for Materials Characterization, National Chemical Laboratory* Division of Organic Synthesis, National Chemical Laboratory**
○K Manoj* R G Gonnade* M M Bhadbhade* M S Shashidhar**
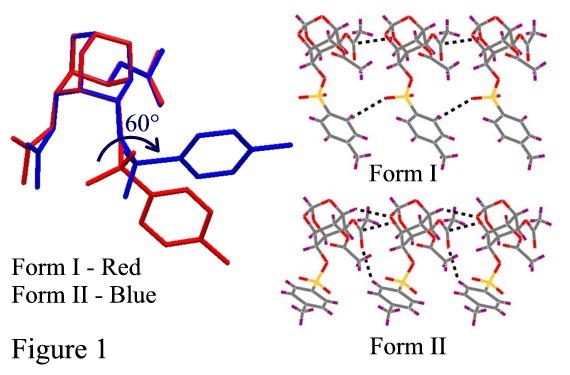
Polymorphism, the ability of a molecule to exist in more than one crystal form, has great implications in chemical industries especially in drugs and pigments. We have reported polymorphic1 and pseudopolymorphic2 behaviour in various myo-inositol derivatives. The title compound (1) showed polymorphic modifications under different crystallization conditions. Compound 1 gave orthorhombic, Pbca crystals (Form I) when crystallized from chloroform-petroleum ether mixture, whereas crystals from ethyl acetate-petroleum ether mixture were monoclinic, P21/c (Form II). Crystal structure analysis revealed that the two forms are conformational polymorphs with C6-O-tosyl group adopting two different conformations (Figure 1). An interesting feature in both the forms is the formation of an almost isostructural string linked by O (orthoester)C=O (acetate) interactions, the deviation arising from different C-HO contacts made by the tosyl group in the two forms (Figure 1). These one-dimensional rows, however, are stitched differently to yield dimorphs.
References:
(1) Gonnade, R. G.; Shashidhar, M. S.; Bhadbhade, M. M. Chem. Commun. 2004, 2530.
(2) Manoj, K.; Gonnade, R. G.; Bhadbhade, M. M.; Shashidhar, M. S. Cryst. Growth & Des. 2006, 6, 1485.
