Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology* College of Science and Engineering, Iwaki-Meisei University, Iwaki, Japan** Asahi Kasei Corporation, Japan***
○M. Mominul Hoque* Ella Czarina M. Juan* Satoru Shimizu* Hiroshi Kondo* M. Tofazzal Hossain* Kaoru Suzuki** Shigeyuki Imamura*** Tamotsu Yamamoto*** Takeshi Sekiguchi** Akio Takenaka*
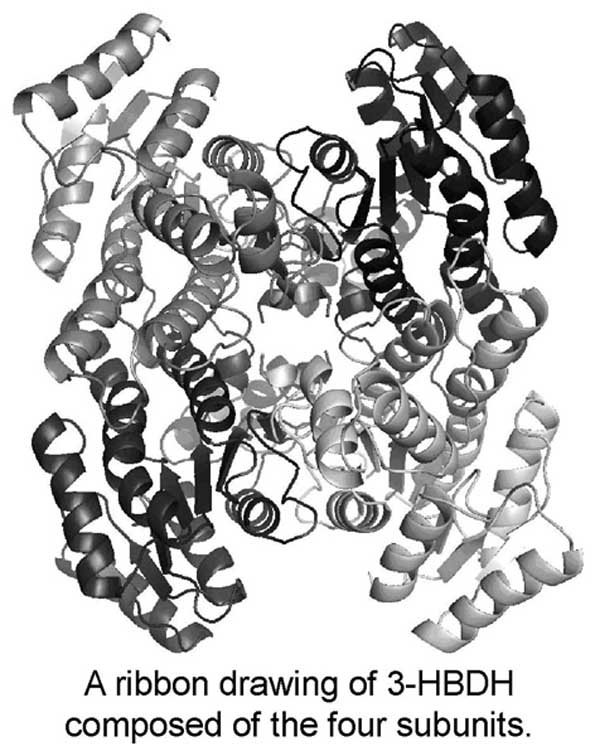
3-Hydroxybutyrate dehydrogenase (3-HBDH) is an NAD-dependent enzyme for reversible conversion between D-3-hydroxybutyrate and acetoacetate. These ketone bodies are important energy sources, but their excess level causes ketoacidosis. 3-HBDH is useful for diagnostic analysis of ketone bodies in diabetes mellitus. To elucidate the reaction mechanism, we started X-ray analyses of 3-HBDH from Alcaligenes faecalis and its complex with NAD. Two crystal forms (I and II) of 3-HBDH and an NAD-containing crystal (III) were obtained by the hanging drop vapor-diffusion method at 293 K. Diffraction data were collected at 100 K with synchrotron radiation at PF(Tsukuba, Japan). Crystal data are a=77.3, b=118.8, c=118.7Å, β=93.8° and P21 for I, a=119.0, b=121.4, c=165.3Å and P21212 for II, and a=165.5, b=165.9, c=152.9Å, and C2221 for III. The preliminary structure of I was solved by the molecular replacement method, and the atomic parameters have been refined at 2.19 Å resolution to R=22% and Rfree=29%. The asymmetric unit contains two tetrameric enzymes composed of the four subunits arranged with non-crystallographic 222 symmetry. Each subunit has a Rossmann fold for NAD binding.
